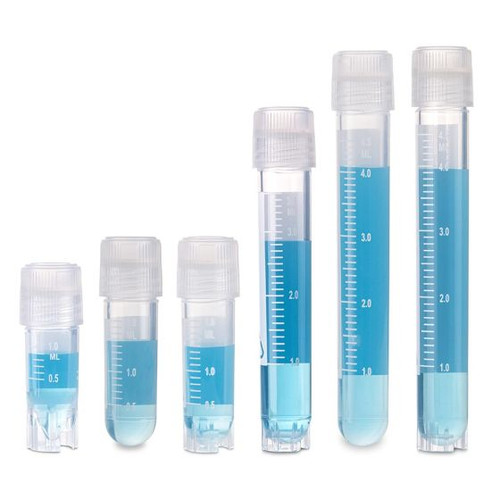
Product Description
The Globe Scientific CryoClear™ vials are designed for the cryogenic storage and transport of biological specimens at temperatures as low as -196ºC. The vials feature an innovative screw cap that is co-molded with a layer of thermoplastic elastomer (TPE) to provide the leak-proof benefits of an O-ring, while eliminating the risk of contamination and sample loss associated with the use of separate O-rings.
Features
- Unique barcode printed on each vial for automated data collection, accurate sample inventory or to conceal the sample’s identity,
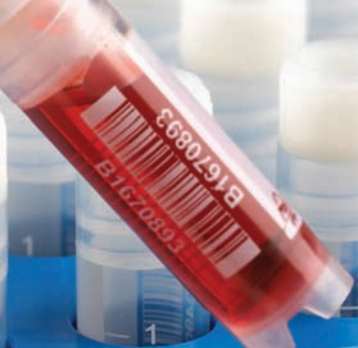
- The caps utilize a star shaped cavity to work with automated capping/decapping equipment,

- White writing surface for specimen identification,
- Printed graduations for accurate measurements,
- Vials and caps are autoclavable,
- Screwcaps are molded with a TPE layer to provide a leak-proof seal,
- Produced from medical grade raw materials that will not discolor after re-sterilizing,
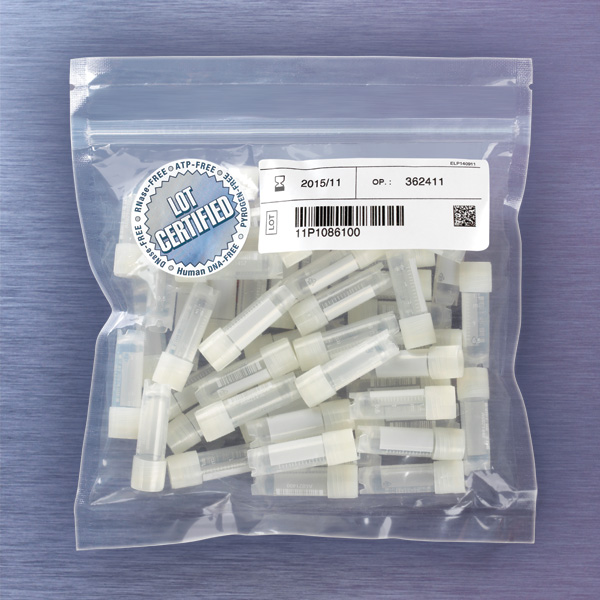
- Tamper evident bags, resealable with lot traceable labelling,
- Self-standing tubes interlock in Globe workstation racks.
Specifications
- Produced from medical grade polypropylene, polyethylene and thermoplastic elastomer (TPE),
- Non-Pyrogenic, Non-Cytotoxic, Non-Mutagenic,
- RNase, DNase, ATP, and Human DNA free,
- Heavy Metals Free,
- BSE/TSE Free (Ingredients of animal origin)
- Not to be used in the liquid phase of liquid nitrogen,
- Packaged as 50 vials in a tamper evident resealable bag, or in box of 10 bags (500 pcs.)

- Internal Threaded CryoClear Vials
Part Number |
Description |
|
GS 3001 |
1.0mL, Conical Bottom, Self-Standing |
|
GS 3002 |
2.0mL, Round Bottom, Self-Standing |
|
GS 3003 |
2.0mL, Round Bottom |
|
GS 3004 |
4.0mL, Round Bottom |
|
GS 3005 |
4.0mL, Round Bottom, Self-Standing |
|
GS 3006 |
5.0mL, Round Bottom |
|
GS 3008 |
5.0mL, Round Bottom, Self-Standing |
- External Threaded CryoClear Vials
Part Number |
Description |
|
GS 3010 |
1.0mL, Conical Bottom, Self-Standing, |
|
GS 3011 |
2.0mL, Round Bottom, |
|
GS 3012 |
2.0mL, Round Bottom, Self-Standing, |
|
GS 3013 |
3.0mL, Round Bottom, Self-Standing, |
|
GS 3014 |
4.0mL, Round Bottom, Self-Standing, |
|
GS 3015 |
5.0mL, Round Bottom, Self-Standing, |
- Material:
- Vial: Medical/FDA Polypropylene (PP)
- Cap: Medical/FDA Polyethylene (PE) co-molded with a thermoplastic elastomer (TPE) layer
- Sterilization: Beta radiation for sterility assurance level (SAL) level of 10-6,
- Temperature range: -196º to +121ºC
- Mold release agents: None used in manufacturing
- Maximum psi: 14.5 psi
- Maximum Relative Centrifugation Force (RCF): 17530 xg
To calculate your centrifuge RCF, use instrument RPM and spin radius (r) as input for by the following equation:
RCF = 11.2 × r (RPM/1000)2
- Printing on vials resistant to the following chemicals:
- Isopropyl alcohol: 5-100%
- Butanol: 100%
- DMSO: 10-20%
- Bleach: 5%
- Acetic acid: 10%
- Hydrochloric acids: 10%
- NaOH: 10%
- Product Certifications (Certificate of Analysis available upon request.)
- Manufacturing environment: Produced, assembled and packaged in a ISO 7 cleanroom (Class 10,000) in accordance with quality guidelines ISO 13485:2004, ISO 14644, ISO 14698 (Federal Standard 209), ISO 14000 and ISO 18000
- Material: Virgin raw materials are tested according to the “United States Pharmacopia” (USP) and with the “drug master file” (DMF) at the FDA Certified under USP Class VI
- CONEG: This product meets CONEG requirements and therefore does not contain heavy metals in the color concentrate
- Sterilization: Sterilized in accordance with EN ISO 11137
- DNase, RNase and pyrogen free: Each lot certified by an independent laboratory to be free of DNase, RNase and pyrogens
- Human DNA and ATP free: Each lot certified by an independent laboratory to be free of Human DNA and ATP
- Non-cytotoxic: This product is certified to be free of cytotoxins
- Non-hemolytic: This product is certified to be free of articles causing hemolysis
- In vitro Diagnostic Medical Device: Complies with Directive 98/79/EC of the European Parliament and the Council of 27 October 1998 on in vitro diagnostic medical devices
- Leak proof: Vials are certified leak proof at a pressure of 95 kPa (0.95 bar, 14.5 psi) by applying a closure force of not less than 8 cNm
- IATA (International Air Transportation Association): Can be used as a primary receptacle for the transport of diagnostic specimens as outlined by the IATA Dangerous Goods Regulations, Part 6.3,5
Optional Accessories
- Cap Insert for CryoClear™ Vials in assorted colors and are ideal for color coding and sample identification. (Click Here to Order)

- Picker for CryoClear vials for the safe and convenient removal of frozen vials from storage boxes. (Click Here to Order)

- BioBOX™ Storage Boxes for CryoCLEAR vials (Click Here to Order)
---

 US Dollar
US Dollar
 AUD - AUSTRALIA
AUD - AUSTRALIA
 JPY -JAPAN
JPY -JAPAN
 ARA - ARGENTINA
ARA - ARGENTINA
 ZAR - SOUTH AFRICA
ZAR - SOUTH AFRICA
 TWD - TAIWAN
TWD - TAIWAN
 CAD - CANADA
CAD - CANADA
 MXN - MEXICO
MXN - MEXICO
 BZD- BELIZE
BZD- BELIZE
 CLP - CHILE
CLP - CHILE
 BRL - BRAZIL
BRL - BRAZIL
 PKR - PAKISTAN
PKR - PAKISTAN
 INR - INDIA
INR - INDIA
 UAH - UKRAINE
UAH - UKRAINE
 GBP - GREAT BRITAIN
GBP - GREAT BRITAIN
 RUB - RUSSIA
RUB - RUSSIA
 CHF - SWITZERLAND
CHF - SWITZERLAND
 CNY - CHINA
CNY - CHINA
 ILS - ISRAEL
ILS - ISRAEL
 EUR - EURO
EUR - EURO
 SAR - SAUDI ARABIA
SAR - SAUDI ARABIA
 SGD - SINGAPORE
SGD - SINGAPORE
 THB - THAILAND
THB - THAILAND
 MYR - MALAYSIA
MYR - MALAYSIA
 PHP - PHILIPPINES
PHP - PHILIPPINES
 IDR - INDONESIA
IDR - INDONESIA
 NZD - NEW ZEALAND
NZD - NEW ZEALAND
 SEK - SWEDEN
SEK - SWEDEN
 DKK - DENMARK
DKK - DENMARK
 NOK - NORWAY
NOK - NORWAY
 TRY - TURKEY
TRY - TURKEY
 KRW - SOUTH KOREAN REPUBLIC
KRW - SOUTH KOREAN REPUBLIC
 PLN - POLAND
PLN - POLAND
 CZK - CZECH REPUBLIC
CZK - CZECH REPUBLIC